
The resulting ions may, however, react with each other to form a precipitate. When two soluble compounds are dumped into water, they will both dissociate. Let's revisit a precipitation reaction to make sure we know what is going on. We will consider each of these in turn through the rest of this chapter.

Long experience with various compounds in the lab give us a set of rules: certain ions (monatomic and polyatomic) always form precipitates when combined with specific ions others never form compounds.

Here is the question: if we combine the solutions, will AD or BC form a solid compound? We already know AB and CD will not (we have the dissociated ion solutions to prove it).

Consider two ionic compounds, AB and CD, which both dissociate completely in water: Some compounds form solids no matter what the solute/solution ratio is. If no solid occurs at this concentration, the compound is considered soluble. 1 M, where M stands for Molar: we will go into detail about this next week).
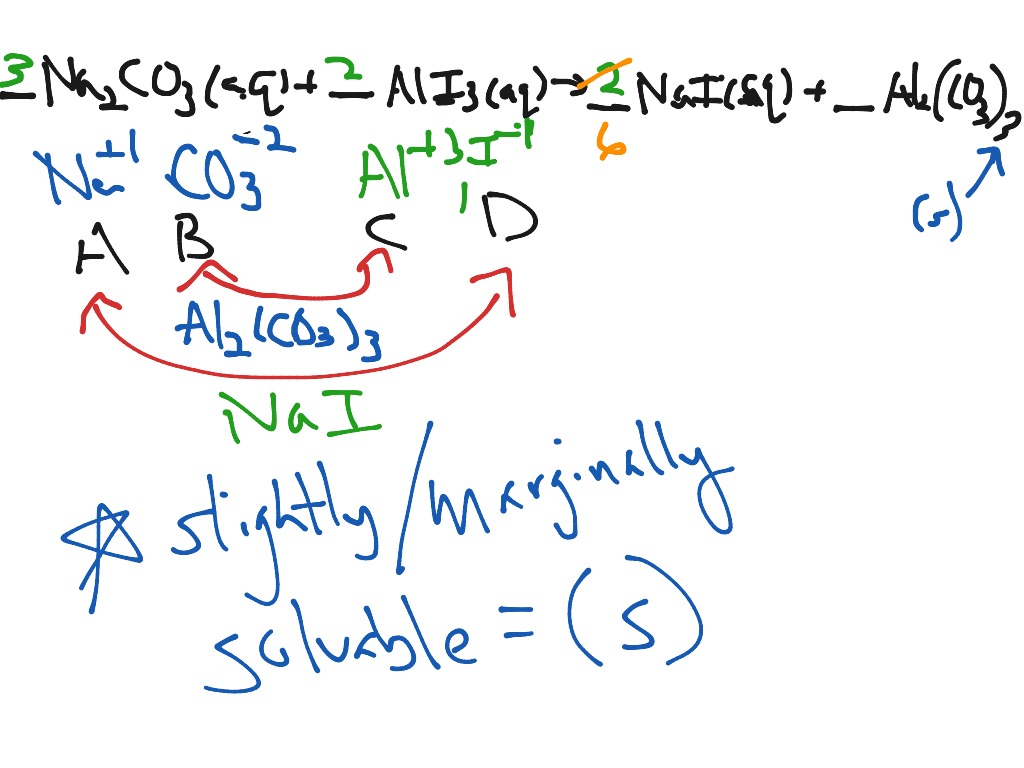
For this reason, the solubility of all compounds is determined at a standard concentration (.1 moles of solute/liter of solution, or. It is possible to put so much solute in a solution that even if the compound is considered soluble, there will still be solid compound present because no more can dissolve in the solution. When table salt dissolves in water at a specific concentration, there are only ions present. To put it bluntly, they come apart (dissociate) in water: Precipitation reactions involve ionic compounds which have completely dissociated in aqueous solution. Most reactions depend on the extent to which the solutes compounds dissolved in the water solvent can dissociate and interact. Reactions in water are extremely important in living cells, electrical cells, and geological processes, so they are the focus of much chemical investigation. Optional Website Reading Characteristics of Reactions in Aqueous Solutions.Characteristics of Reactions in Aqueous Solutions.Aqueous and Precipitation Reactions Outline


 0 kommentar(er)
0 kommentar(er)
